Data from an early phase trial published in Nature found that Parkinson disease patients treated with UB-312 experienced significant improvements in motor functions and daily living.

Data from an early phase trial published in Nature found that Parkinson disease patients treated with UB-312 experienced significant improvements in motor functions and daily living.

Approval of Sofdra marks the first chemical entity to be approved by the FDA to treat primary axillary hyperhidrosis.
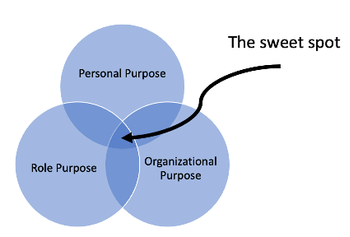
Findings from hundreds of interviews of exceptional pharma leaders around the world.

The platform, named Pharmacy Match, will connect markets for specialty drugs.

A Q&A with Korn Ferry’s co-managing director of the Global Education Practice, Kenneth L. Kring.
In an interview with Pharm Exec Associate Editor Don Tracy, Pedro Valencia, VP, Solid Tumor Pipeline Strategy & Execution, AbbVie, discusses using novel cancer biomarkers to develop potential first-in-class antibody drug conjugates (ADCs) and the promise that they offer.

Approval of Skyrizi marks the first IL-23 specific inhibitor approved for both ulcerative colitis and Crohn disease of similar severity, according to AbbVie.

The platform will use machine-learning and generative AI to provide insurers with a variety of options and increase automation in their processes.
Approval of Keytruda combination for patients with primary advanced or recurrent endometrial carcinoma is based on results from the NRG-GY018/KEYNOTE-868 clinical trial, which was Phase III trial to evaluate an anti-PD-1 immunotherapy plus chemotherapy combination in patients with two distinct tumor types.
Approval for Capvaxive was based on results from three Phase III trials, which found Capvaxive to be beneficial preventing invasive pneumococcal disease and pneumococcal pneumonia in both vaccine-naïve and vaccine-experienced adults.

The CDMO’s facilities will be used to accelerate the production for a treatment of nAMD.

Health insurance executives say that digital technologies are getting members more involved in the healthcare system.
The approval of Blincyto was based on results from the Phase III E1910 clinical trial, which found that the treatment significantly improved overall survival in CD19-positive, Ph-negative B-cell ALL compared to chemotherapy alone.
The approval of Imfinzi was based on the DUO-E Phase III trial, which indicated that in combination with chemotherapy, the drug reduced the risk of disease progression or death by 58% in mismatch repair deficient endometrial cancer.

This is the first time in 20 years that CalPERS has changed its sole health plan provider.
Data presented at the European Hematology Association Congress indicated that patients with chronic lymphocytic leukemia treated with Imbrivica had a significantly longer median progression-free survival compared to patients treated with chlorambucil.

Gabi Hanna, MD, CEO and Co-Founder of Lamassu Pharma talks about a novel oncology platform and the link between science and patient care.
Approval for Augtyro was based on results from the Phase I/II TRIDENT-1 trial, which demonstrated significant response rates in both tyrosine kinase inhibitor (TKI) naïve and TKI-pretreated patients.

The court ruled that the plaintiffs did not have standing to sue in this situation.

According to the company, the additional software makes it algorithm more accurate.

Novel approaches against chronic inflammation could potentially prevent certain inflammatory diseases and neurological conditions.

AstraZeneca’s Farxiga has previously been approved by the FDA as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus, in addition to indications for heart failure.

In an interview with Pharm Exec Associate Editor Don Tracy, Pedro Valencia, VP, Solid Tumor Pipeline Strategy & Execution, AbbVie, discusses data presented at ASCO from ABBV-400 and ABBV-706 in multiple studies.
The device will be 4g-enabled and connect with the company’s remote monitoring platform.

The two companies are combining digital platforms to provide prescribers more options to monitor patients’ medication adherence.

Investigators followed a group of people born from 1990-1996 to assess whether the association between preterm birth and income differs according to family socioeconomic status at birth.

Aliada Therapeutics' chief scientific officer, John Dunlop, PhD, discusses the novel platform.

The cobas is classified as a four-in-one molecular test that can identify SARS-CoV-2, influenza A, influenza B, and respiratory syncytial virus from a single nasopharyngeal or anterior nasal swab sample.

Hypermedica and Ro announced platforms to help patients find access to affordable weight loss medications.

Approval for Kevzara was based on controlled studies, pharmacokinetic data from adults with rheumatoid arthritis, and pediatric-specific studies on pharmacokinetics.