
Congress and our nation’s public health officials should take the current moment to self-reflect and resolve challenges with public confidence in our public and scientific institutions.

Congress and our nation’s public health officials should take the current moment to self-reflect and resolve challenges with public confidence in our public and scientific institutions.

Advancements in periodontal disease could make way for expansions in the dental space and other medical fields.

JAMA study aims to discover how weight loss differs between patients receiving tirzepatide compared with semaglutide among a clinical population of overweight of obese adults.

Avexitide has received Breakthrough Therapy Designation by the FDA for post-bariatric hypoglycemia, congenital hyperinsulinism, and more.

Empara’s virtual assistant is the latest attempt to use AI to simplify the user experience in the healthcare industry.

If you wish for your team to be diligent, innovative, and collaborative, you must first exhibit these traits yourself

According to the Complete Response Letter, the FDA has requested additional information on the manufacturing process and the type 1 diabetes indication for insulin icodec before completing its review.
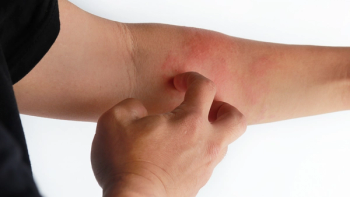
Acquisition of Yellow Jersey Therapeutics includes access to NM26, a potential treatment for atopic dermatitis.

JAMA study evaluates changes to out-of-pocket costs and utilization of type 2 diabetes medications once patients reach the age of 65 years.
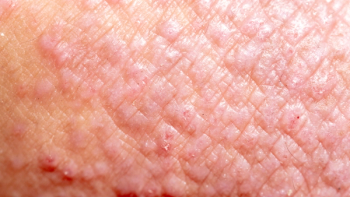
Zoryve is a steroid-free, once-daily treatment shown to offer rapid disease clearance and significant itch reduction for patients with mild to moderate atopic dermatitis.

A Harvard Business School Healthcare Alumni Association Q&A with Penn Center for Innovation’s Michael D. Moisel, MS, MBA.

The compounding pharmacy acquired Taylors Pharmacy and Key Compounding Pharmacy.

Body Vision’s AI-powered LungVision image system earned a place in Vizient’s innovative technology program.
Approval of the Mynx Control Venous vascular closure device was based on results from the ReliaSeal trial, which demonstrated 100% procedural and device success in cardiac ablation procedures.

Executives in the life sciences and medical device industries see promise in artificial intelligence (AI) and medical technologies, but must also navigate the changing healthcare ecosystem, varied stakeholder needs, and the impact of AI on market access and commercialization strategies.
Acquisition of Morphic is aimed to enhance treatment options for inflammatory bowel disease and expand Lilly's gastroenterology portfolio.

In an interview with Pharm Exec Associate Editor Don Tracy, Kathy Lee-Sepsick, Founder, CEO, Femasys, discusses results of the Femaseed pivotal trial, Fembloc birth control, and how the outcome of the 2024 United States presidential election could impact women's health.
Results from the Phase III STREAM Stage 2 study show the efficacy and safety of an all-oral bedaquiline-containing regimen for multidrug-resistant pulmonary tuberculosis.

The Vabysmo prefilled syringe is designed to simplify administration for retina specialists, improving the treatment experience for both physicians and patients with wet age-related macular degeneration, diabetic macular edema, and macular edema following retinal vein occlusion.

The company announced that it had received certification from BSI Medical Devices.

In an interview with Pharm Exec Associate Editor Don Tracy, Kathy Lee-Sepsick, Founder, CEO, Femasys, offers a description of how Femaseed works to support women who struggle with infertility.

MTX325 is in Phase I trials and is believed to be able to modify the course of the disease.
Funding from the Biomedical Advanced Research and Development Authority (BARDA) is expected to support the late-stage development and licensure of a pre-pandemic vaccine targeting the H5 influenza virus.

The platform is similar to other recent digital platforms in that it is designed to reduce drug costs.

The company announced that it has partnered with Adena Health and The Ohio State University Health Plan Solutions.

Kisunla is the first amyloid plaque-targeting therapy that allows for stopping treatment upon plaque removal, company says.
Results of the Phase III CARTITUDE-4 study showed that treatment with Carvykti achieved a more significant improvement in overall survival (OS) compared to standard therapies.
The Fast Track designation for INZ-701 was based nonclinical pharmacology data and preliminary safety and efficacy data from the ongoing Phase I/II trial of INZ-701 in adults with ABCC6 Deficiency.

Patients who received PrimeC reportedly saw significant impact to the disease’s progression.

As previously announced, Michael assumed the role on July 1.