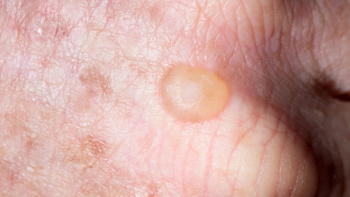
Klisyri is now available in a 350 mg package size as a topical treatment for actinic keratosis on the face or scalp.

Klisyri is now available in a 350 mg package size as a topical treatment for actinic keratosis on the face or scalp.

A Q&A with Egon Zehnder’s Sergio Della Zassa about what biopharma executives and search firms are looking for in terms of table stakes skill sets and “unicorn” experiences from top talent in the field.

Arexvy receives expanded indication to include adults aged 59 years and younger to prevent RSV lower respiratory tract disease.
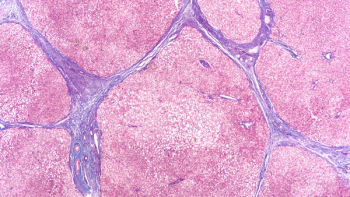
Survodutide is the first glucagon/GLP-1 receptor dual agonist to show a high level of benefit in treating liver fibrosis associated with metabolic dysfunction-associated steatohepatitis.

Both partnerships will bring new research and healthcare opportunities to Abu Dhabi.

The partnership is described as the first of its kind.

Approval of Rytelo could provide patients with lower-risk myelodysplastic syndromes with extended periods without the need for red blood cell transfusions to alleviate symptomatic anemia.
According to the manufacturer, the LFR-260 features advanced technology that allows patients to compare multiple prescriptions simultaneously, eliminating the need for traditional refraction methods.

The new agency will operate under the name Deerfield Group, and will also include Verge Scientific Communications.
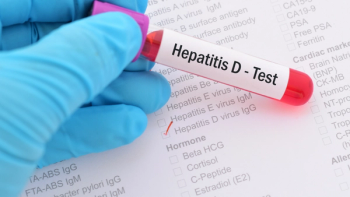
Data published in the New England Journal of Medicine from Gilead’s Phase IIb MYR204 found the bulevirtide/ pegylated interferon alfa-2a combination was more effective in achieving undetectable HDV RNA compared to bulevirtide 10 mg monotherapy in the treatment of chronic hepatitis D.
Acquisition of Elsie Biotechnologies is expected to strengthen GSK’s research capabilities in modulating gene expression through the use of oligonucleotide.

The organization announced greater support for access and services for the community.

Anat Ashkenazi will leave the company at the end of July to pursue a career outside of pharma.

A similar application was also submitted to the EMA for the expanded use of Prezcobix to treat children aged 6 years and older with HIV.

In a majority vote, the Psychopharmacologic Drugs Advisory Committee cited concerns regarding the data presented for MDMA as a treatment for post-traumatic stress disorder.

The industry has struggled to recruit talent for years, and the challenge is only getting more difficult. Pharma companies need to recruit differently.
Reportedly, data from the IMROZ study marks the first time an anti-CD38 monoclonal antibody combined with standard-of-care therapy has demonstrated significant improvement in progression-free survival for newly diagnosed transplant-ineligible multiple myeloma.
After three years of study, the V940 and Keytruda combination demonstrated sustained benefits in recurrence-free survival and distant metastasis-free survival in patients with high-risk melanoma following complete resection.

Delivering better patient outcomes hinges on innovation and adaptability.
BD announced that it will pay cash to acquire the advanced monitoring solutions company.
Results of the ADRIATIC trial indicated that treatment with Imfinzi after standard-of-care concurrent chemoradiotherapy improves overall survival and progression-free survival in patients with limited-stage small cell lung cancer.
Results of the phase II supported collaborative study with Memorial Sloan Kettering Cancer Center (MSK) show that 100% of participants achieved a clinical complete response when treated with Jemperli for locally advanced rectal cancer in patients with mismatch repair deficient status.

The drug had previously been submitted, but required resubmission due to production issues.

The platforms are described as being first-in-category for the pharma industry.
Results of a Phase III trial indicated that Scemblix was more effective than standard-of-care tyrosine kinase inhibitors in treating newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase.

Under terms of the deal, Akili will become a wholly owned subsidiary of Virtual Therapeutics, with Akili shareholders receiving $0.4340 per share of common stock in cash.
In an interview with Pharm Exec Associate Editor Don Tracy, Karen Rodriguez-Lorenc, Global Program Head of Linvoseltamab, Regeneron talks about the possibility of linvoseltamab becoming a frontline treatment for multiple myeloma (MM) as well as the company's VelociSuite technology.

Approval of Onyda XR marks the first liquid non-stimulant medication for attention-deficit hyperactivity disorder to hit the market in the United States.
Breyanzi, a CD19-directed CAR T-cell therapy, granted fourth FDA approval to treat a distinct subtype of non-Hodgkin lymphoma.
Submission for the FDA approval of zanidatamab was based on promising data from the Phase IIb HERIZON-BTC-01 clinical trial in patients with previously treated, unresectable, locally advanced, or metastatic HER2-positive biliary tract cancer.