
How “Contractual Genericization” could revitalize pharma and reframe the public conversation.

How “Contractual Genericization” could revitalize pharma and reframe the public conversation.

IQVIA has announced the launch of OCE Optimizer, which will look to help life sciences companies reach HCPs more effectively.



Gilead's pricing of COVID drug remdesivir is a reminder of pharma's tenuous path forward as it seeks a more holistic image makeover.









While scientists are striving to discover and produce safe and effective vaccines to halt the coronovirus outbreak, anti-vacciners are revving up misinformation campaigns.


Investors welcome biopharma sector initiative.




Welcome to the new look and branding of Pharmaceutical Executive-reimagined to better reflect you, our audience, and expand on the content you've long trusted us to deliver.

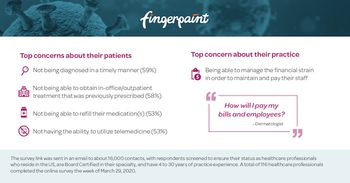







ZS offers advice to ensure reps can stay engaged with their customers during these times of changing needs and constraints.