
While most pharma companies are in partial production using generators, few are operating at 100% capacity, writes Jill Wechsler.

While most pharma companies are in partial production using generators, few are operating at 100% capacity, writes Jill Wechsler.

Agency leaders go slow in weighing changes to DTC ads and off-label marketing.

FDA is evaluating whether limiting warnings in TV commercials to the most serious adverse effects might be more informative for consumers than the current laundry lists of potential side effects.

The extensive volume of pharmaceutical manufacturing in Puerto Rico is driving FDA efforts to assess facility damage and logistical problems to ensure continued supply of critical medicines. Jill Wechsler reports.
Jayachandra Reddy and Rishit Thakkar discuss the challenges facing early entrants in the Non-alcoholic Steatohepatitis market.

While biopharma companies have pressed hard for clarity on the data required to gain market approval of biosimilars that can be filled by a pharmacist without prescriber pre-approval, the progress towards "interchangeability" has been slow. Jill Wechsler reports.

Can the pharma industry truly achieve a patient-centric supply chain? How some clinical trial logistics companies are racing ahead in making the patient a key link in the chain.

Despite delay in enforcement of Drug Supply Chain Security Act's new serialization requirement, getting compliant now is key.


Drug pricing, right-to-try, opioids, and OTC improvements still on legislative agenda.
As regulators lower evidentiary requirements for approval to speed development and review of new drugs for unmet medical needs, payers are demanding more data to justify price premiums. Companies need to be strategic in how they navigate these complexities, write Bengt Anell, Sangeeta Budhia and Richard Macaulay.

With a new consultation from the UK’s Department of Health proposing changes to the Statutory Scheme for Pricing of Branded Medicines, what could this mean for negotiation of a successor to the 2014 Pharmaceutical Price Regulation Scheme (PPRS)? Leela Barham reports.

The federal Open Payments program is finally reaching its goal-to halt the adoption of multiple “transparency” initiatives by states and local governments with a national system for collecting and disclosing data on industry payments. Will it make a difference?

Four strategic takeaways from this year's that are important for pharma oncology leaders.

As more states approve the use of medical marijuana-and its rising profile as potential opioid alternative-industry and FDA are stepping up their focus on developing cannabis-derived therapies.

The growing toll requires new assessment of risks, expanded provider education, and more scrutiny of marketed products.

The agency’s new commissioner unveiled new digital health pilot programs to help navigate regulatory requirements.


Value frameworks are now "sexy". In the US, no fewer than five have emerged since around 2015. Leela Barham discusses how the value assessment landscape is evolving.

The UK's proposed Accelerated Access Review is being dogged by delay and disappointment, writes Leela Barham.
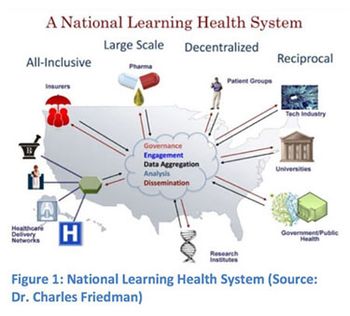
The FDA's Mary Ann Slack outlines how the Agency is working to adopt electronic practices by initiating efforts to simplify the submission process for new drug and generic drug applications.
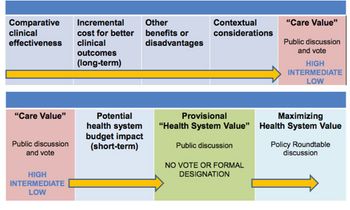
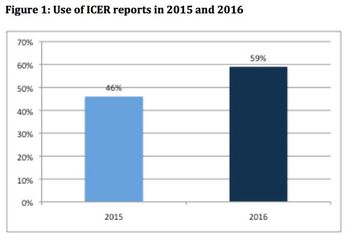
With the US Institute for Clinical and Economic Review (ICER) looking like it is here to stay, Leela Barham considers how its value framework is evolving.
In the second of her articles on US value assessment, Leela Barham reviews the emergence of multiple frameworks and looks at their potential impact.

To gain better alignment of coverage and risk, evidence-based arguments are critical during the underwriting process.

Three messages that resonated from this year’s annual meeting of the Drug Information Association.