
As we wrap up the week in San Francisco, here’s PharmExec's brief rundown of some of the top tweets of the week from JP Morgan and Biotech Showcase.

As we wrap up the week in San Francisco, here’s PharmExec's brief rundown of some of the top tweets of the week from JP Morgan and Biotech Showcase.

Click on the links below to download Pharmaceutical Executive's media kits.


With its new healthcare insurance scheme, Jaminan Kesehatan Nasional (JKN), aiming to cover all citizens by 2019, next year will see Indonesia emerge as the most promising emerging market for pharma investment, according the Frost & Sullivan’s Reenita Das.

Building an effective drug forecasting model today requires a revamped approach.

At first glance Obamacare developments for the American Rx industry in 2015 appear fairly benign. However, digging deeper suggests that each, in its own way, could cause various actions with potential for substantial uncertainty for the U.S. pharmaceutical industry next year. If your response to this profound observation was, “Really, what’s new?” - read on.

Pharm Exec profiles Express Scripts' Chief Medical Officer, Dr. Steve Miller, whose rise to prominence as an articulate critic of drug industry prices symbolizes the growing clout of America's largest pharmacy benefit manager.


US researchers and regulators continue to support the use of randomized clinical trials to test potential treatments and vaccines to combat the Ebola virus, despite objections that this approach is unethical and unfair to vulnerable populations

The Food and Drug Administration is moving fast to implement the drug compounding provisions of new Drug Quality and Security Act (DQSA), issuing new guidance to spur registration by outsourcing facilities just days after President Obama signed the new bill into law.

For Moe Alsumidaie, the most concrete definition for patient centricity is “a dynamic process through which the patient regulates the flow of information to and from him/her via multiple pathways to exercise choice that is consistent with his/her preferences, values and beliefs.

Not all companies are big, small companies are scared, and myths drive behavior, stated Kay Holcombe, Senior Vice President, Science Policy at Biotechnology Industry Organization (BIO).
Cleveland Clinic's Pick of Top 10 Advances for 2015.

The pharmaceutical and biotechnology industries, like all industries, are facing industry-specific changes coupled with disruptive events in the external global environment.

The innovation debate (Why isn’t there more or it? How much should we pay for it? Just what is it anyway?!) remains a lively one in the UK. Lord Saatchi’s Medical Innovation Bill is just one part of this debate.

The battle against hepatitis C (HCV) is a relatively new, and highly dynamic, one.

“Only those who risk going too far can possibly find out how far they can go,” said T.S. Elliot. Cancer Research UK (CRUK) followed this advice when it decided to have its Center for Drug Development (CDD) adopt a risk-based monitoring (RBM) approach across its entire portfolio of clinical trials.

I can’t help imagining the cocktail party of 2018 as a scene from one of those a low-budget sci-fi film aired late at night. Imagine: A living room packed with people too busy to chat with each other as they balance their drinks with one hand and monitor their sleeves of devices and smartphones with the other - emailing, texting checking their wearable ECGs (electrocardiograms); glucose monitors and even insulin pumps.

The past year has brought significant changes not only in regulatory mandates and guidances but also regarding a broader overall emphasis on coordination of information and processes.

Shire plans to relocate more than 500 positions to Massachusetts from its Chesterbrook, PA, site and establish Lexington, MA, as the company’s US operational headquarters.

The Tufts University Center for the Study of Drug Development (CSDD) released its latest research on the costs and timeframes required for launching a new drug.
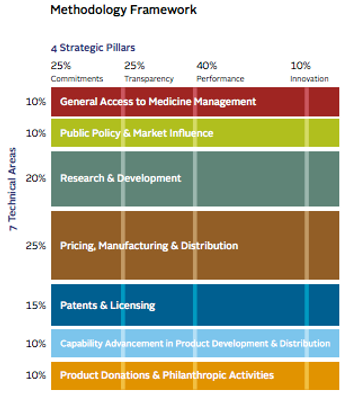
Last week, the Access to Medicine Foundation released its 2014 Index rating the performance of companies in broadening access to medicines in low and middle-income countries.

The European Medicines Agency (EMA) has published its revised policy on handling declarations of interests for scientific-committee members and experts.

CytRx announced on Tuesday that FDA gave notice of a partial clinical hold for trials for its oncology candidate aldoxorubicin.

The Pharmaceutical Care Management Association (PCMA), an organization that represents the nation’s leading pharmacy benefit managers (PBMs), which administer prescription drug plans for over 210 million Americans, released a new white paper investigating FDA’s influence on drug prices and competition in the pharmaceutical marketplace.

A cool $2.6 billion is the going rate to develop and gain market approval for a drug, according to the recent study by the Tufts Center for the Study of Drug Development (CSDD).

The pharmaceutical market is beginning to show signs of stability, with a review of 30 leading companies highlighting combined revenues of $718.7 billion in 2013, down just 0.2% from 2012, according to GlobalData.

While flipping through my emails recently, I froze before an image that appeared on my screen. An old Rx colleague had sent me a graph, mapping out the 2014 U.S. sales achieved by Gilead’s Hep C drug, Sovaldi. It was stunning.

Peter O’Donnell’s recent Applied Clinical Trials blog, ‘Who’s in charge of Medicines in Brussels?‘, has been overtaken by history, with the departure of Guido Rasi from his post of Executive Director of the European Medicines Agency.

In response to a letter the European Medicines Agency received from the European Ombudsman on Oct. 27, 2014, the agency is clarifying its policy on commercially confidential information.