
Data from the ENHANCE-3 trial of magrolimab in combination with azacitidine plus Venclexta showed futility and an increased risk of death in patients with acute myeloid leukemia.

Data from the ENHANCE-3 trial of magrolimab in combination with azacitidine plus Venclexta showed futility and an increased risk of death in patients with acute myeloid leukemia.

Results from the Phase III CheckMate -77T trial show that Opdivo (nivolumab) plus chemotherapy followed by surgery and adjuvant Opdivo produced statistically significant and clinically meaningful improvements in event-free survival in patients with resectable stage IIA to IIIB non-small cell lung cancer.
Vepdegestrant (ARV-471) is a noval PROTAC ER degrader found to harness the body’s own natural protein disposal system to eliminate disease-causing proteins.
BioNTech SE and Duality Biologics' next-generation antibody-drug conjugate is being evaluated for patients with platinum-resistant ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer previously administered one to three systemic treatment regimens.
The FDA assigned a Prescription Drug User Fee Act action date of June 7, 2024, to an application that would expand the indication of Arexvy to include adults 50-59 years with an increased risk of respiratory syncytial virus-related lower respiratory tract disease.
UV1 plus ipilimumab (Yervoy) and nivolumab (Opdivo) produced a statistically significant and clinically meaningful survival improvement in patients with unresectable malignant pleural mesothelioma.

Novartis rescinds rights to to develop and commercialize multi-tyrosine kinase inhibitor dovitinib due to a material breach by Allarity for lack of financial payment.
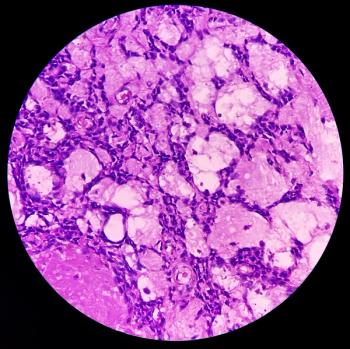
The biologics license application for afamitresgene autoleucel, an engineered T-cell receptor drug, was assigned a PDUFA date of August 4, 2024.
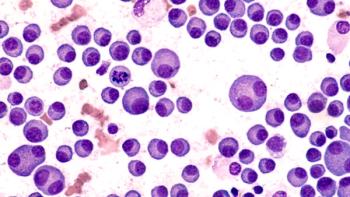
Darzalex Faspro has previously been approved by the FDA for eight indications in multiple myeloma.

CAR T-cell therapy Breyanzi (lisocabtagene maraleucel) to be evaluated in patients with relapsed or refractory follicular lymphoma and mantle cell lymphoma following treatment with a Bruton tyrosine kinase inhibitor.

This is Takeda's second approval in chronic inflammatory demyelinating polyneuropathy this month after the FDA approved HyQvia to protect against relapse of neuromuscular disability and impairment in this patient population.
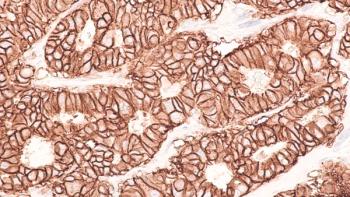
Enhertu has been approved by the FDA for indications in breast cancer, non-small cell lung cancer, and gastroesophageal junction adenocarcinoma.
Atypical antidepressant linked to severe adverse effects and death as a result of misuse.

The FDA previously approved Dupixent in May 2022 to treat eosinophilic esophagitis in patients aged 12 years and older.

FDA leadership notes that the overall rate of secondary T-cell cancers among patients administered CAR T-cell therapies appears to be low, even if all reported cases are assumed to be related to treatment.

The expanded indication for Zynrelef now includes soft tissue and orthopedic surgical procedures.
Theratechnologies said it will address the FDA’s complete response letter and will continue to seek approval for the new formulation of tesamorelin for the treatment of lipodystrophy in patients also diagnosed with HIV.

Severe hypocalcemia was found to be more common among patients with advanced chronic kidney disease with mineral and bone disorder who are taking Prolia for osteoporosis.
New FDA guidelines require manufacturers to add boxed warnings to CAR T-cell therapy products.

The device can produce AI-assisted readings.

The FDA was unable to approve the Biologics License Application for zolbetuximab because of deficiencies found in a third-party manufacturing facility for the drug, which is under evaluation to treat patients with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma.

Supplemental Biologics License Application would convert the accelerated approval granted to Tivdak (tisotumab vedotin-tftv) to a full approval for patients with recurrent or metastatic cervical cancer with disease progression on or after first-line therapy.
Rinatabart sesutecan (Rina-S; PRO1184) is under evaluation for the treatment of patients with FRα-expressing high-grade serous or endometrioid platinum-resistant ovarian cancer.

In an interview with Pharmaceutical Executive associate editor Don Tracy, Ashley Gaines, VP, head of breast cancer franchise, discusses newly approved Truqap.
Trial data show 89Zr-DFO-girentuximab was more effective than traditional PET/CT imaging in identifying malignant renal cell carcinoma lesions.